Hardly a day seems to pass without new headlines about the ever-growing costs – in lives, health and funds – of diabetes. Researchers at Imperial have developed a new technique using both mice and human tissue to help them better understand the mechanisms underlying the disease. Now they are using these insights to seek improved treatments for people living with diabetes.

If we want to find a cure, something we are still some way from doing, we really need to understand pancreatic islet biology.
Dr Victoria Salem
Around one in 10 adults worldwide – more than 500 million people – live with diabetes.1 Prevalence in the UK is rising rapidly, with the latest estimate, published in April, putting case numbers at more than 5 million. The disease directly causes an estimated 2 million deaths per year worldwide, and is a major cause of blindness, kidney failure, heart attacks, stroke and lower limb amputations.
The key to understanding and potentially finding a cure for diabetes lies in clusters of cells called pancreatic islets. These tiny groups of cells produce hormones including insulin, which is key to keeping blood glucose levels within a normal, safe range. Diabetes occurs when pancreatic islets are either attacked by our immune systems (type 1), or become damaged through overwork and, as a result, fail to make enough insulin (type 2).
Until a few years ago, these clues to the complexities of beta cell activity could only be shown in experiments that could not accurately replicate the natural functioning of pancreatic islets. That’s because they were often carried out on islets removed from human cadavers or animals, without the continuous blood supply and neural inputs they get in a living body.
The insights gained from these types of experiments were useful but limited in how they could be applied clinically. One way to study pancreatic islets ‘in vivo’ is to repeatedly take blood samples from humans or animals, however this too has its limitations. “We used to infer pancreatic islet function indirectly by taking regular peripheral blood samples, but this involves taking it a long way from where the insulin is released and so it doesn’t accurately reflect what is going on,” says Victoria. “And even the most advanced modern imaging technologies don’t have the resolution to be able to directly image these tiny islets deep within the pancreas.”
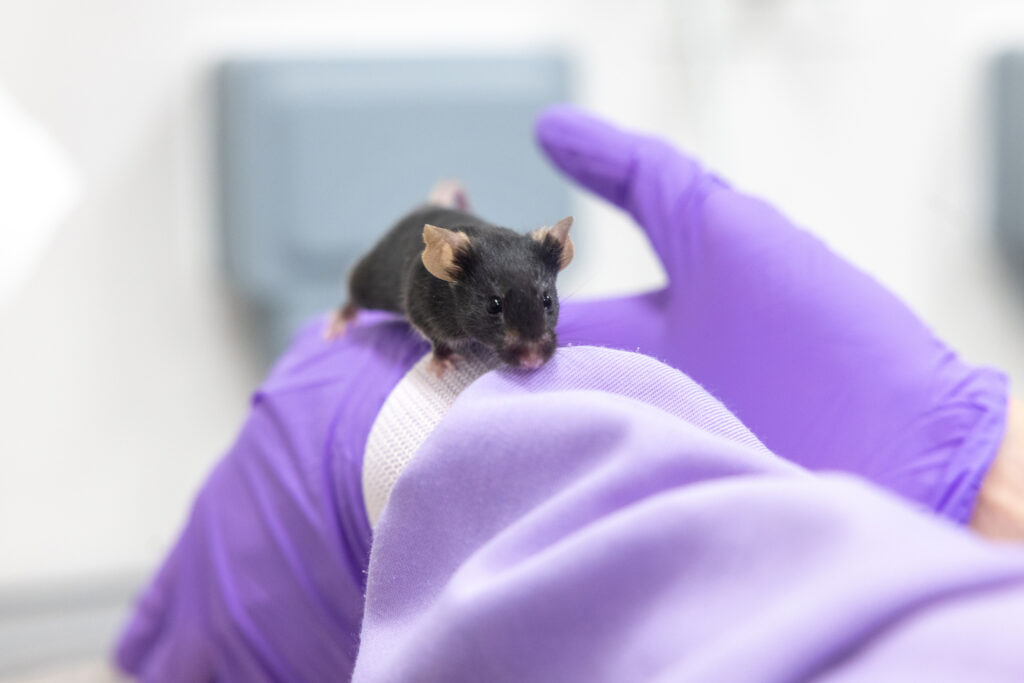
Scientists have long used a method in laboratory mice involving transplanting tissue into a space between the cornea and the iris called the anterior chamber of the eye (ACE) as a way to better study it. Three Researchers at the Karolinska Institute in Stockholm, Sweden, successfully did this with murine pancreatic islets, and showed that these developed a blood supply and used the technique to study how they responded to a variety of interventions.
Victoria and her team went a stage further by genetically engineering mice so that beta cells in their pancreatic islets produce a fluorescent chemical when they release insulin. They also engineered pancreatic islets from human bodies donated for medical research to do the same thing.
The group transplanted both the mouse and human islets into the ACE of mice, and observed them developing a blood supply. “Efforts to find a cure for diabetes have been held back by the difficulties involved in studying pancreatic islets in their natural, living environment. Our method is the only way, to date, that allows us to image and interrogate the pancreatic islet and see its changing behaviour over time in response to different medicines or stimuli, in a live animal.”

