Replacement – the use of non-animal research techniques instead of animal models – is perhaps the most fundamental and the most challenging of the 3Rs. The experience of researchers at Imperial shows that novel approaches to replacement can yield significant breakthroughs in research.
In some situations, animals can be replaced in medical research by working with human tissues collected during medical procedures or after death. But obtaining these human tissues is not always straight forward.
This was the experience of Richard Reynolds, Professor of Cellular Neurobiology in the Faculty of Medicine. For many years he used animal models in his work on multiple sclerosis, trying to find out how the neurons in this degenerative condition become damaged and eventually die.
“I realised that I needed to verify some of the things I was finding in those animal models using human tissue, to make sure that they were truly representative of what was happening in the human condition,” he recalls. “But when I tried to get this tissue, there weren’t any good resources.” In the end, he and his colleagues set up their own tissue bank, collecting brains and spinal cords from people who had died after having multiple sclerosis. This was later extended to Parkinson’s disease and for comparison they also collected brains not affected by these two conditions.
Eighteen years on, the Multiple Sclerosis and Parkinson’s Tissue Bank at Hammersmith Hospital is one of the largest in the world. It holds tissue from 1,600 brains and close to 12,000 people around the UK have agreed to donate to the bank when they die. This resource is not just used by Imperial’s researchers, but is shared with scientists around the world.
Having access to this material has transformed Professor Reynolds’ research. First of all, it showed that the animal model he started out with was a poor fit for the research question he was working on. Then it provided an alternative to the animal model. “We started to spend much more time getting as much information from the human tissue as we could, to develop our hypothesis about why the cells were dying, in a very detailed way,” he says.
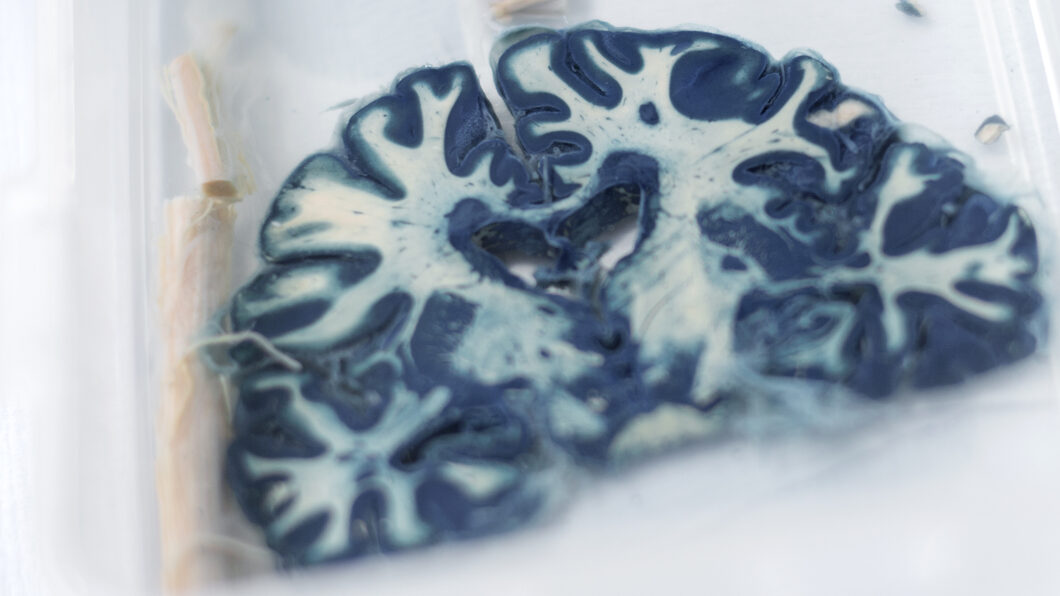
One difficulty is that each sample of human tissue represents just one moment in the development of the disease, after death, whereas animal studies allow the progress of the disease to be followed over time. That can be partly overcome if the tissue bank covers a broad range of case histories. “Although we can’t study how a single patient changed over time, we have a population who died at various stages, with various severities and length of disease. That allows us to do some sort of longitudinal study in time, effectively replacing what we might otherwise do in animals,” Professor Reynolds explains. “In the end, it has replaced at least 50 per cent of our animal work.”
He also thinks that the science is sounder this way. “If we are trying to develop therapies for human diseases, at least some of our studies need to involve the tissue that is affected by those diseases.”
Some animal work was still necessary, however, in this case to check that the new hypothesis stood up in a living system. But thanks to the studies on human tissue, a much more effective animal model could be designed to do this, reducing the number of animals used. “We’ve shown that our ideas about the mechanism probably are right, and we’ve identified a number of drug targets,” says Professor Reynolds. The next step is to go back to the human tissue to make sure that these targets are present early enough in the development of the disease to make drugs acting on them effective in halting its progress. Once that is confirmed, the search for potential drugs begins.